
A rapidly evolving and complex trial ecosystem has made it more difficult to efficiently manage and oversee clinical trials. Rave CTMS (Clinical Trial Management System) improves speed and efficiency for the oversight of studies, countries, and sites through intelligent automation and workflow management. The Medidata Platform streamlines the clinical operations ecosystem so data transfer between Rave EDC , Medidata Detect , and Rave eTMF is automated, eliminating manual data re-entry and enabling user-centric workflows that bridge applications.

Automated workflows streamline manual processes for key activities, and dashboards focus you on what matters most. Milestones, tasks, issues, and document status are tracked in one place. Data is entered once and used everywhere, eliminating information silos and providing tremendous efficiency for site and monitoring staff.
Scale More Easily ExpandRave CTMS scales from early phase through late phase trials. It also integrates with your existing technology and workflows.
Reduce Avoidable Startup Delay Expand 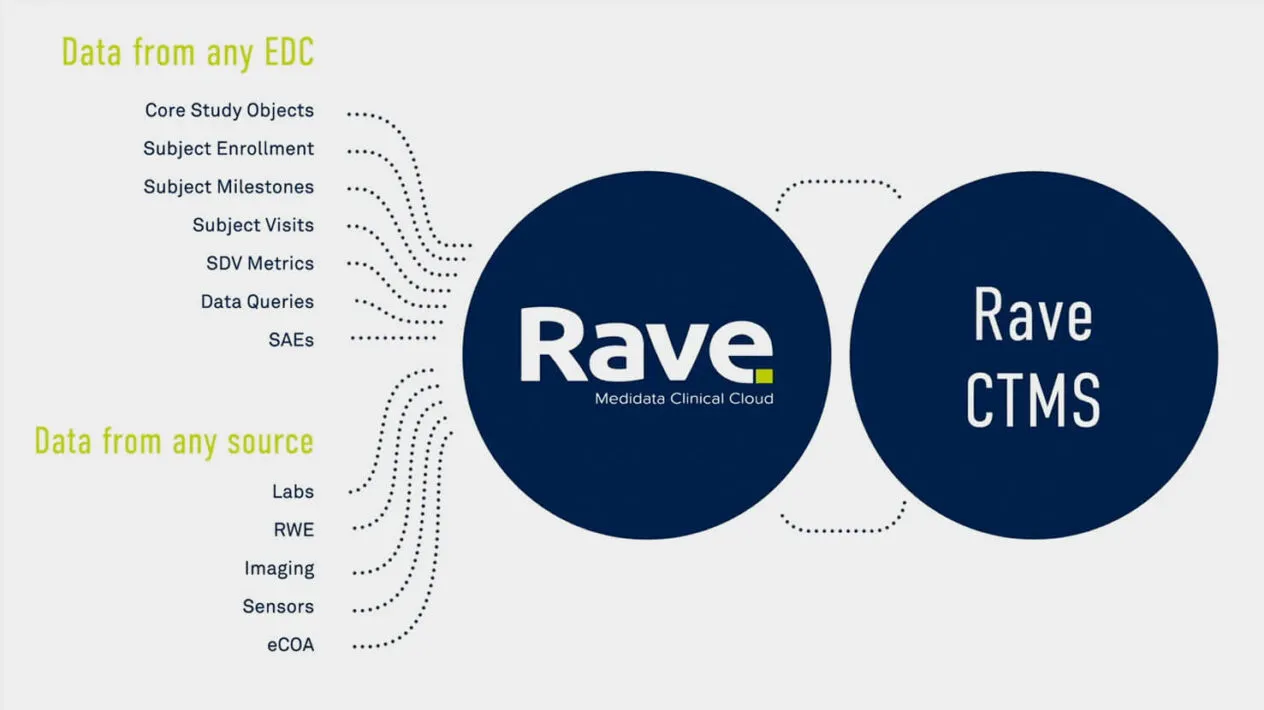
Study startup activities are embedded within Rave CTMS and Rave eTMF , where site-specific required milestones and tasks are tracked in one place. A closed-loop system of site activation activities and issue management eliminates multiple status spreadsheets and tracking reports, saving you time and effort.
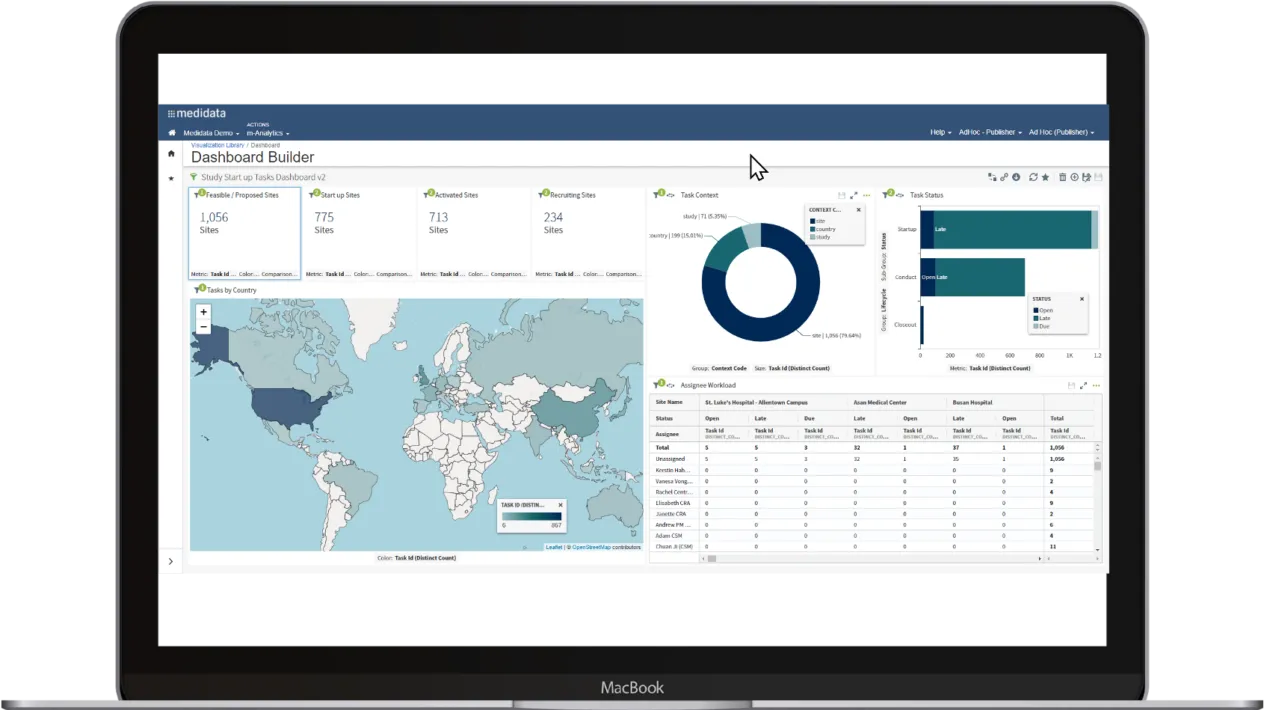
Efficiently manage the entire study with data automatically populated from any EDC for dashboards, forecasts, and analysis. With Rave CTMS Visual Analytics, create intuitive visuals by combining Rave CTMS data across studies into a single visualization, report, or dashboard.
Unified Document Management Expand 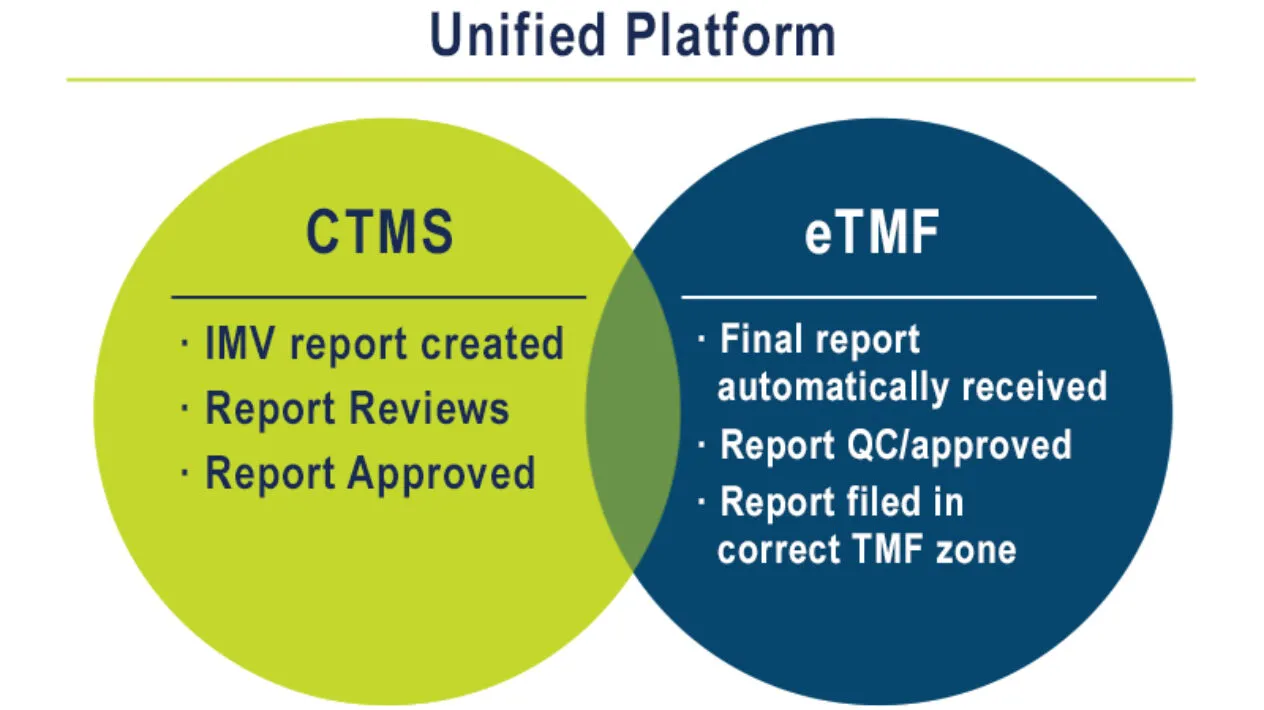
Rave CTMS is tightly integrated with Rave EDC and Rave eTMF to optimize collaboration with sites. Users upload documents once and the eTMF is automatically updated with data and content. Data is auto populated into monitoring visit reports, which are then automatically filed to the TMF.
Visibility Into Risk Expand 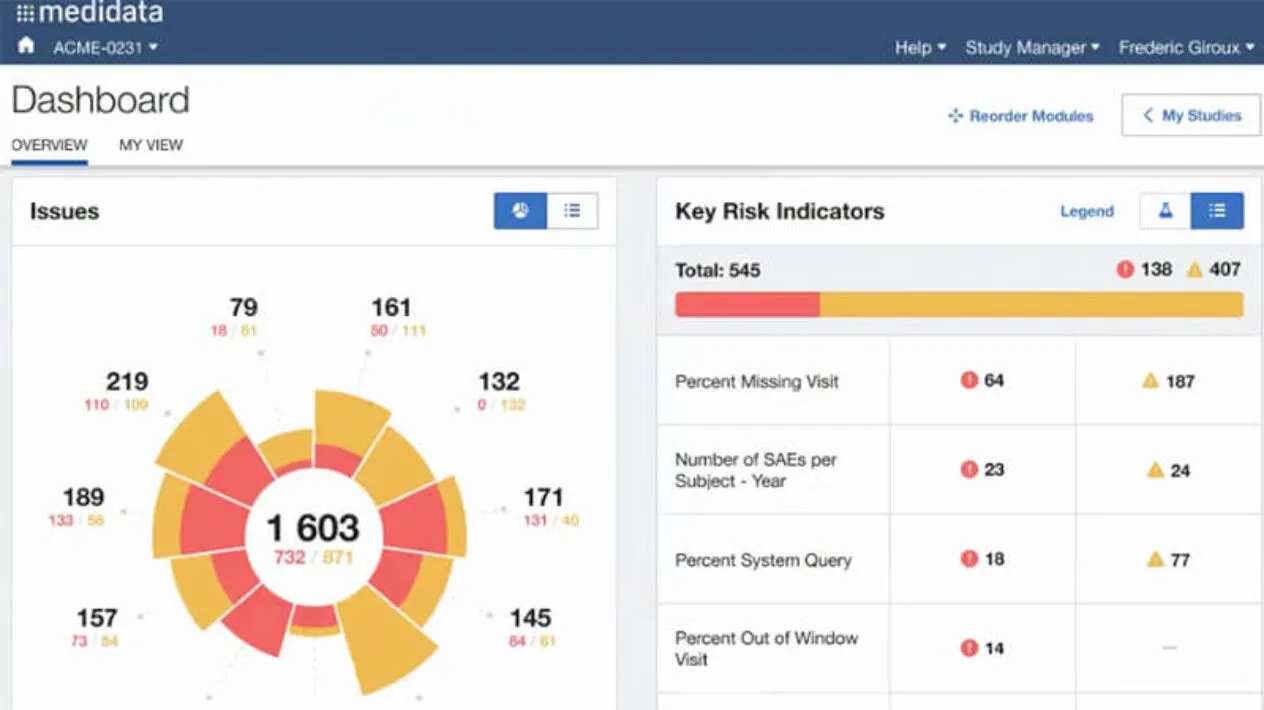
Your CRAs and study managers are empowered with analytical insights driven from key risk indicators (KRIs) to ensure patient safety and study accountability.
Risk-Based Monitoring Expand 
Easily move to a paradigm where interactions with sites are purpose-driven and with intention, focused on critical data and processes. Rave CTMS is integrated with RaveTSDV (Targeted Source Data Verification) , Medidata Detect , and Medidata Remote Source Review .
Rave eTMF is a global, secure collaboration platform that easily manages Trial Master File content so it is always contemporaneous with the study.
Learn moreMedidata Detect is a powerful data and risk surveillance tool for centralized monitoring, powered by automated statistical algorithms and machine learning. Detect improves your study data quality and ensures patient safety throughout the trial.
Learn more 
Rave CTMS, unified on the Medidata Platform , is your transactional hub to natively and intelligently connect workflows, deliver data-driven insights, and foster collaboration – all powered by unified data capture.
Visual Analytics Fact Sheet Expand 
See how Visual Analytics for Rave CTMS can drive efficient workflows, enable collaboration across teams and gain oversight of your study progress. With powerful visualizations and easy-to-use tools, you can take back control of your study.
Enterin Case Study Expand 
Learn how fast-growing biopharmaceutical company Enterin implemented Rave CTMS to enable their resource-constrained team to work more effectively.
Catalyst Case Study Expand 
Learn why and how Catalyst Clinical Research personnel identified a need for a scalable CTMS solution that would grow with their organization and support their customer throughout the drug development process, and why they selected Rave CTMS.
© 2024 Medidata - All rights reserved
We, Medidata, use cookies to give you the best experience on our websites by: measuring their audience and improving their performance, by providing you with content and proposals that correspond to your interactions, by serving ads that are relevant to your interests and by allowing you to share content on social networks.
Your preferences will be kept for 6 months and you can change them at any time by clicking on the "Manage my cookies preferences" link in the footer of each webpage. To learn more about how this site uses cookies, please visit our privacy policy.
We may collect information using “cookies.” Cookies are small data files stored on the hard drive of your computer or mobile device by a website. We may use both session cookies (which expire once you close your web browser) and persistent cookies (which stay on your computer or mobile device until you delete them) to provide you with a more personal and interactive experience on our Site.
Complete details can be found in our Privacy Policy.
Necessary Cookies Always EnabledNecessary cookies are essential and are used to provide you with services available through Medidata website. For instance, these cookies allow Medidata to remember your choices about cookies preferences, to record your interface customization trackers e.g. for the choice of language used by the website. Necessary cookies are enabled by default and cannot be switched off. To see the list of the cookies used for this purpose, click here .
functionalFunctional cookies are used to provide you with contents and proposals that correspond to your interactions. They may consist of information logged on your device or recorded as you navigate through Medidata website. These cookies also allow us to analyze site usage so we can measure and improve performance. To see the list of the cookies used for these purposes, click here.
advertisingAdvertising cookies are used to enable Medidata and its trusted Medidata business stakeholders to serve ads that are relevant to your interests. The intention is to display ads that are relevant to you.
To see the list of the cookies used for this purpose, click here.